Magnetic resonance imaging (MRI) is a non-invasive imaging technique that uses magnetic fields and radiofrequency waves to generate high-resolution anatomical and functional images. Since its clinical introduction in the 1980s, MRI has become an essential tool in neurology, oncology, musculoskeletal medicine, cardiovascular imaging, and abdominal diagnostics, among other fields. This article reviews the physical principles underlying MRI, its main sequences, clinical applications, and emerging developments such as functional MRI, spectroscopy, and artificial intelligence for image analysis.
Physical Principles
MRI is based on the phenomenon of nuclear magnetic resonance (NMR), primarily applied to the hydrogen nucleus (proton), which is abundant in water and fat in the human body.
When the patient is placed within a strong, homogeneous magnetic field (typically 1.5 to 3 teslas), protons align either parallel or antiparallel to the magnetic field. A radiofrequency (RF) pulse at the Larmor frequency is then applied, disturbing this equilibrium. When the RF pulse ceases, the protons return to their baseline state, emitting energy that is detected by the system’s coils.
Two key relaxation times are involved:
- T1 (longitudinal relaxation): the time it takes for the magnetization vector to realign with the main magnetic field.
- T2 (transverse relaxation): the time it takes for transverse magnetization to decay due to loss of phase coherence.
Each tissue has characteristic T1 and T2 values, allowing for distinct contrast between different structures.
Imaging Sequences and Contrast Mechanisms
MRI’s versatility lies in its ability to adjust acquisition parameters to highlight different tissue characteristics.
Common sequences include:
- T1-weighted: ideal for anatomical detail and lipid-rich structures (spinal cord, basal ganglia, liver).
- T2-weighted: emphasizes water content; useful for edema, inflammation, tumors.
- FLAIR: suppresses cerebrospinal fluid; ideal for periventricular lesions.
- STIR: fat suppression; useful for musculoskeletal pathology and soft tissue tumors.
- Diffusion-weighted imaging (DWI): evaluates molecular water motion; critical in acute stroke and tumor cellularity.
- Perfusion and spectroscopy: assess tissue metabolism and vascularity.
Intravenous paramagnetic contrast agents, such as gadolinium, may be administered to assess tissue enhancement and permeability.
Clinical Applications
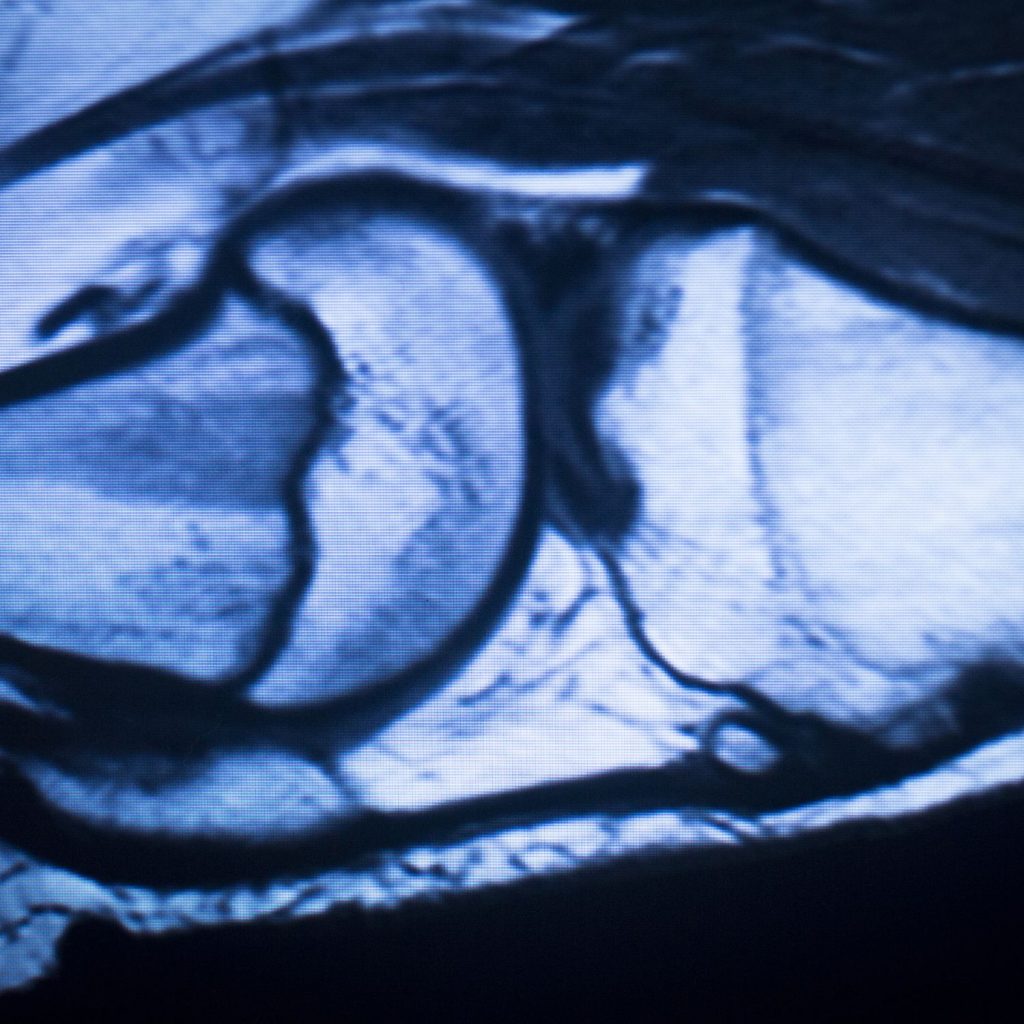
a) Neuroradiology
- Early detection of ischemic stroke via DWI.
- Tumor evaluation in the CNS: characterization, extension, treatment response.
- Diagnosis and follow-up of multiple sclerosis, epilepsy, congenital malformations.
- Assessment of cerebral atrophy in neurodegenerative diseases.
b) Musculoskeletal Imaging
- Evaluation of tendons, ligaments, bone marrow, and cartilage.
- Diagnosis of soft tissue tumors.
- Detection of osteomyelitis and septic arthritis.
- Postoperative and prosthetic joint assessment.
c) Cardiovascular Imaging
- Ventricular function analysis, cardiac masses, myocarditis.
- Congenital heart disease assessment.
- Myocardial perfusion and viability using late gadolinium enhancement.
d) Oncology
- Local and regional staging of tumors.
- Monitoring systemic therapy response.
- Multiparametric MRI for prostate, liver, breast, and uterine cancers.
e) Abdominal and Pelvic Imaging
- Liver lesion characterization: benign vs. malignant.
- Magnetic resonance cholangiopancreatography (MRCP).
- MR enterography for inflammatory bowel disease.
- Evaluation of gynecological disorders (fibroids, adenomyosis, endometriosis).
Advantages over Other Modalities
- No ionizing radiation, making it safe for repeated use.
- Excellent soft tissue contrast.
- High-resolution multiplanar imaging without the need for reconstruction.
- Capability to perform functional and metabolic studies (e.g., diffusion, perfusion, spectroscopy).
Limitations and Contraindications
- Long acquisition times, especially for advanced sequences.
- Claustrophobia and the need for patient stillness, limiting use in pediatric or neurologically impaired patients.
- Contraindicated in patients with non-MRI-compatible pacemakers, cochlear implants, or certain metallic fragments.
- Small risk (though very low) of nephrogenic systemic fibrosis (NSF) related to gadolinium in patients with severe renal impairment.
Emerging Trends and Research Directions
- Functional MRI (fMRI): used in neurosurgical planning, brain mapping, and psychiatric research.
- Artificial intelligence: enhances pattern recognition, noise reduction, and automatic segmentation.
- Magnetic resonance spectroscopy: assesses tissue metabolites in the brain, liver, and tumors.
- Whole-body MRI (WB-MRI): useful for systemic disease assessment, such as multiple myeloma.
- Ultrafast imaging sequences (e.g., Turbo MRI, Zero Echo Time) to improve image acquisition in uncooperative patients.
Magnetic resonance imaging is a complex, versatile, and highly sensitive imaging modality that has transformed diagnostics across multiple specialties. Its ability to provide anatomical, functional, and metabolic information without radiation makes it an irreplaceable tool in modern medicine.
As demand rises and technology advances, the future of MRI lies in further automation, diagnostic precision, and expanded accessibility—positioning it at the core of personalized medicine and translational research.